Determine the Oxidation Number of Each Element in if
H 2 O 2 S 8. Chemical oxidation is carried out by adding reactive chemicals.
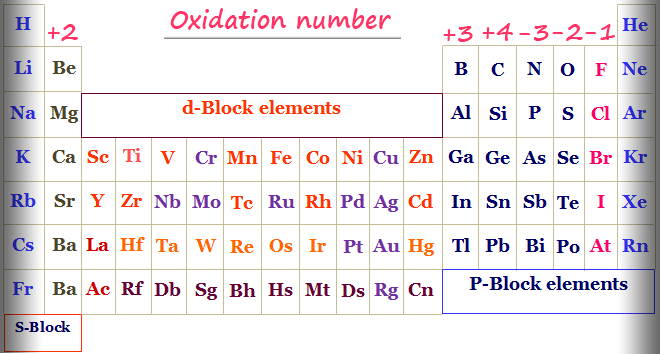
Oxidation Number Periodic Table Elements Definition Rules
In each of those three cases you can determine the oxidation state of manganese by using the known oxidation state of oxygen and the overall charge of the ion when that is the case.
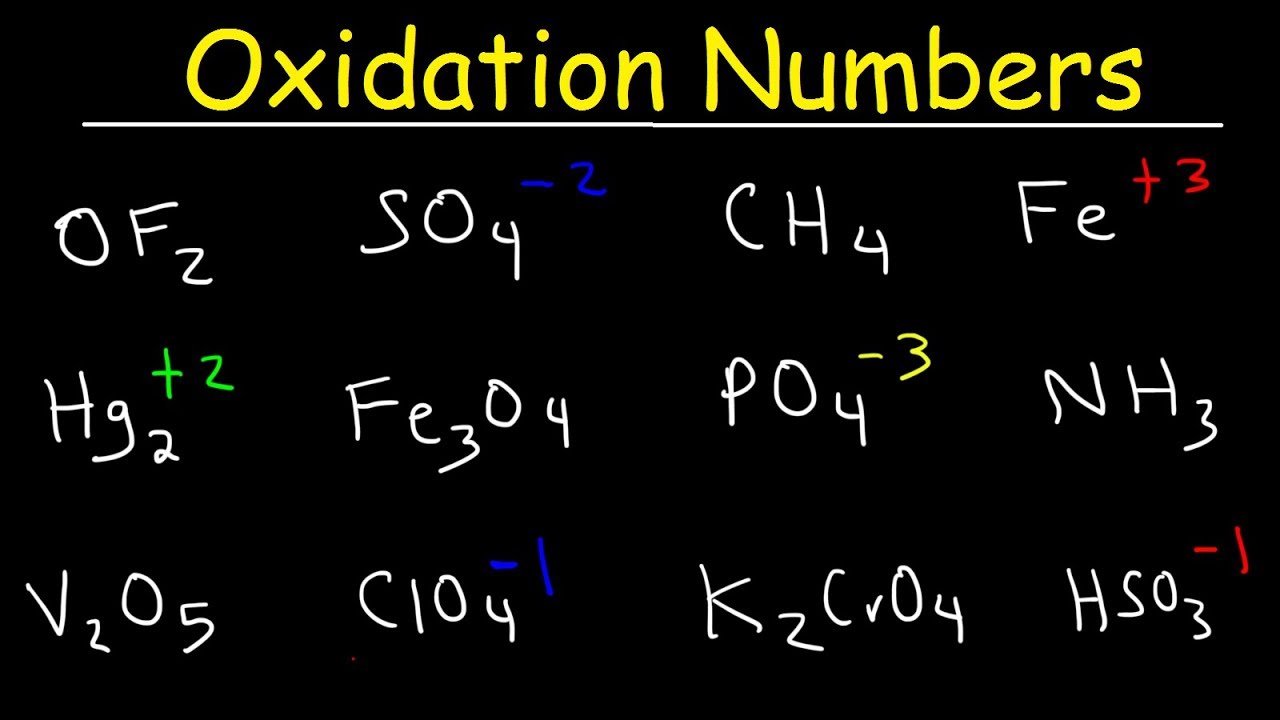
. Chemical oxidation and advanced oxidation processes were discussed in detail in Chapter 6. There are a few rules to keep in mind that help you identify the charges. Oxidation can be accomplished through chemical oxidation or other enhanced oxidation processes for degrading organic contaminants in oily wastes.
All group 1 elements at 1. The simplest way to interpret oxidation number is to think of it as the number of electrons lost or gained by an atom compared to its neutral uncombined form when it reacts to form ions or molecules. The valence charge can be determined by looking at the position of the element on the periodic table.
Transition elements will have Roman numerals in parentheses to indicate their charge. All group 2 elements are 2. The initial concentration of each compound was set at 100.
Oxidation state or oxidation number is a bookkeeping device employed by chemists to help them classify and understand chemical reactions. The oxidation number of a monatomic one-atom ion is the same as the charge on the ion for example. It is predicted that even a 12 oxidation state may be achievable by uranium in the unusual hexoxide UO 6.
The highest known oxidation state is reported to be 9 in the tetroxoiridiumIX cation IrO 4. The oxidation number of an element in its free uncombined state is zero for example Als or Zns. The sum of all oxidation numbers in a neutral.
Determine the valence charge of each element. Na S2 Rule 3. The lowest oxidation state.
Oxygen has a -2 oxidation state in these compounds. So youre dealing with the permanganate ion MnO_4- Here the ion has an overall 1- charge. The oxidation state or oxidation number.
This is also true for elements found in nature as diatomic two-atom elements. The average oxidation state of an element is a fraction such as 8 3 for iron in magnetite Fe 3 O 4.

Calculate The Oxidation Number Of The Underlined Elements In The Following Compounds A Kunder Youtube

Oxidation Numbers Sulphur Exhibits Oxidation Numbers Of 2 0 2 4 And 6 Chemistry High School Chemistry Teaching Science

How To Calculate Oxidation Numbers Basic Introduction Youtube
No comments for "Determine the Oxidation Number of Each Element in if"
Post a Comment